42 Years of Collaborations in Cardiology!
Cardialysis, a premier Cardiovascular Research Organization specializing in Cardiology, offers comprehensive clinical research and cardiovascular core laboratory services. Established in 1983 as a spin-off of the Thoraxcenter, Erasmus Medical Center, it is renowned for its extensive key-opinion-leader network, deep academic ties, and robust methodological expertise. Over the years, Cardialysis has mastered its full-service clinical operations, driven by professionals with academic expertise and advanced skills in managing and executing clinical trials. Collaborating with top global medical device, biotechnology, and pharmaceutical companies, Cardialysis has contributed to over 400 clinical trials, advancing innovative cardiovascular therapies to hospitals worldwide and enhancing patient outcomes and quality of life.
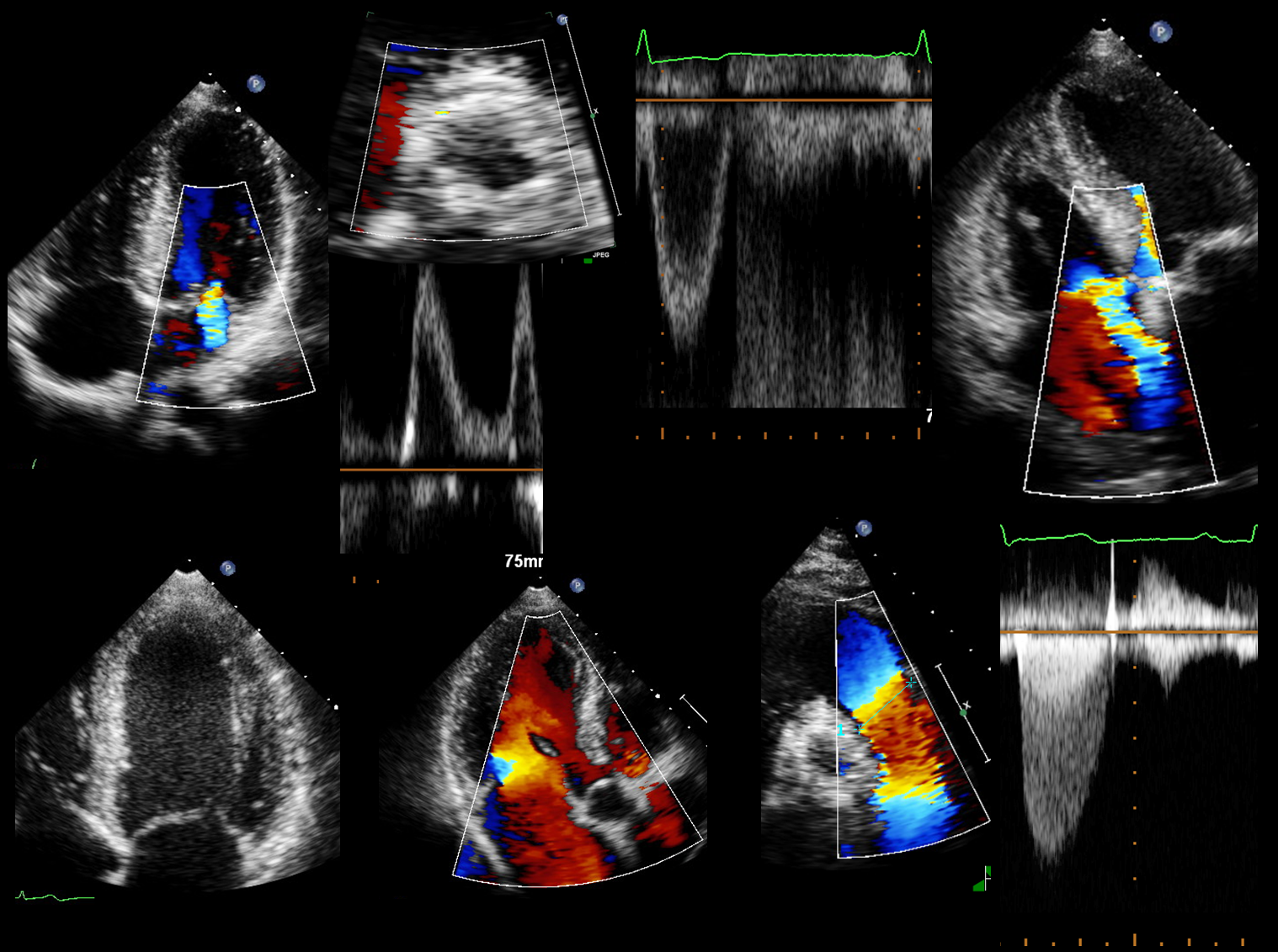
Structural Heart Core Lab
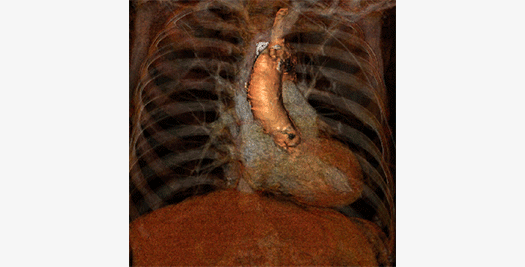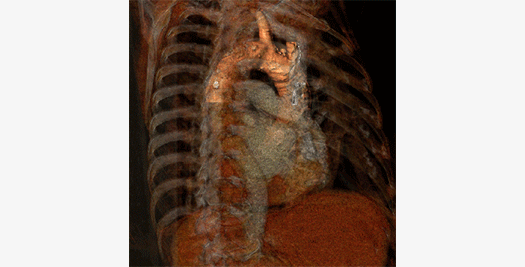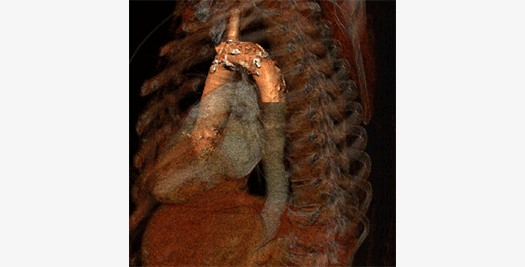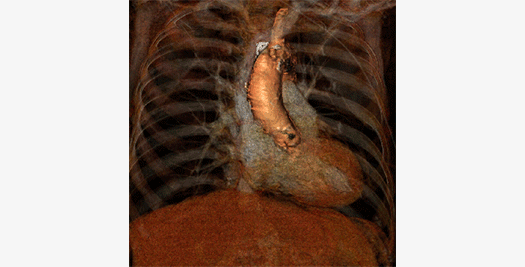
The Cardialysis Structural Heart Core Lab provides the perfect partner in conducting transcatheter therapies clinical trials (e.g. TAVR, TMTT). Over the past few years thousands of imaging data from patients enrolled in several registries and clinical trials have been successfully analyzed and adjudicated by the Cardialysis Core Lab. These data are used for CE mark and FDA submissions.
Investigator Initiated Studies

The European Cardiovascular Research Institute (ECRI) offers a platform for the design and conduct of international investigator-initiated clinical trials. ECRI acts as Sponsor ensuring compliance with ICH GCP guidelines and regulatory standards. Trial activities are executed by Cardialysis alone or in collaboration with renowned research organizations world-wide.
Monitoring Services
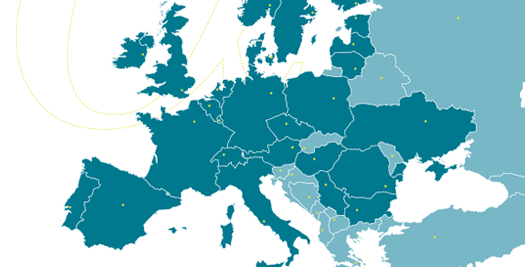
Cardialysis offers a consolidated monitoring network across Europe. Our clinical research associates ensure timely activation of investigational sites, high-quality data review and effective interaction with sites. With >1400 sites monitored and >200,000 patients included, Cardialysis has established a significant track record in site management.