EU-MDR Cardiovascular Collaboratory
The EU-MDR Cardiovascular Collaboratory (EU-MCVC) is an international expert network which brings together European academicians, clinical trialists, and regulatory experts to collaborate with clinical research stakeholders, regionally and globally.
The purpose of EU-MCVC is to create a dynamic and open conversation to continuously achieve an effective implementation of clinical research in Europe with emphasis on navigating the European Medical Device Regulation (EU-MDR). Relevant stakeholders to this collaboratory are cardiovascular research organizations, registry platforms, regulatory bodies, and industry partners.
EU-MCVC Board
- Dr. Ernest Spitzer - Cardialysis & European Cardiovascular Research Institute (ECRI Foundation) (Rotterdam, The Netherlands)
- Prof. dr. David Erlinge - Department of Cardiology, Lund University Hospital (Lund, Sweden)
- Dr. Armando Pérez de Prado - Hospital Universitario de León & EPIC Foundation (Leon, Spain)
- Prof. Jan G.P. Tijssen - Cardialysis & European Cardiovascular Research Institute (ECRI Foundation)

Deliverables 2023-2025
The deliverables of EU-MCVC include:
- Yearly educational meeting
- Develop on-line resources
- Yearly white paper or publication
- Develop the EU-MCVC Registry Network
- Participation in international working groups
Accomplishments 2023-2025
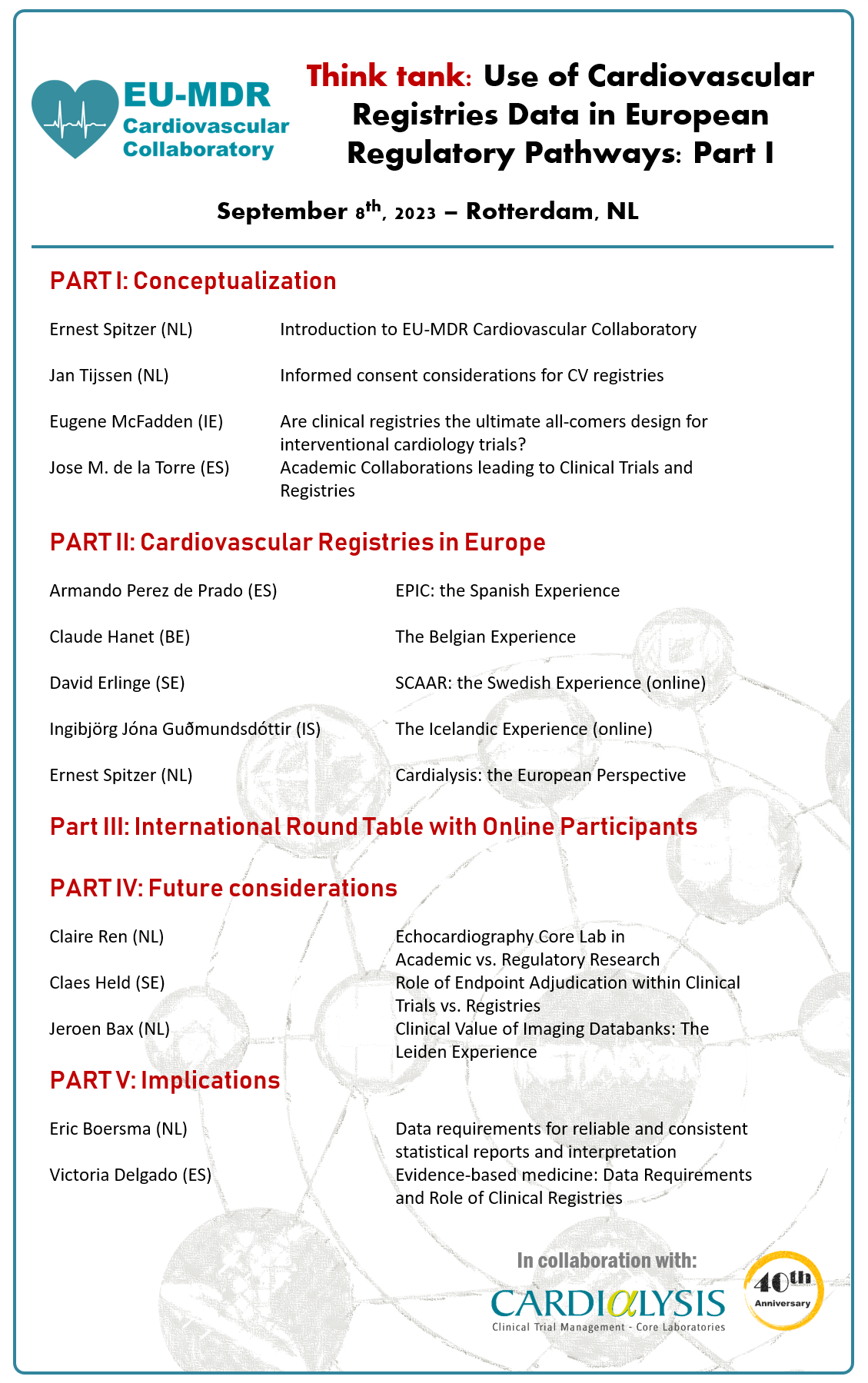
First think tank on Use of Cardiovascular Registries Data in European Regulatory Pathways: Part I, which took place in Rotterdam on September 8th, 2023, including 13 Faculty and 42 registered participants, representing 9 European countries and 12 countries globally.
Faculty Picture in Rotterdam

Next steps: White paper in preparation.