Aims and business model
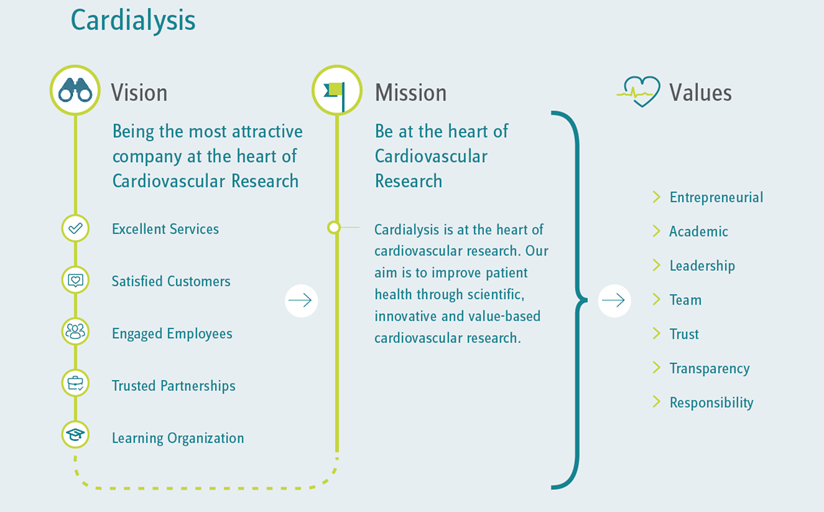
Cardialysis aims to contribute to the evaluation and development of improved therapies for cardiovascular care. We believe that forward-looking clinical research requires a synchronized collaboration between science and management. We bring together world-renowned scientific leaders and effective operational execution, ensuring timeliness, data quality, data integrity, and most importantly patient safety.
To set-up collaborative projects, Cardialysis acts as a pivot between the expertise, skills, and facilities at leading academic research centers and investigational sites in Europe; and Industry sponsors or grant-givers, driving Sponsor-initiated studies or Investigator-initiated studies. Although most renowned for our involvement in cardiovascular devices research with our advanced Core Laboratory capabilities, we are as much involved in drug development.
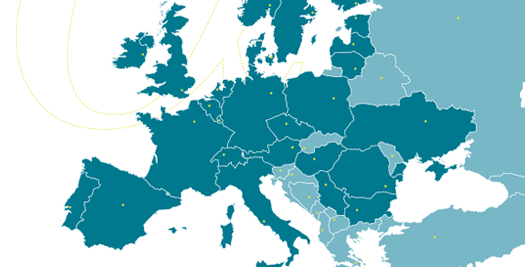
All our clinical research activities are performed within the European regulatory framework and international standards, with access to 1200+ investigational sites throughout Europe.